Researchers Turn Graphite Into Diamond
2014.04.07

Graphite and diamonds are both made from carbon. So can the process turn the former into the latter? Turns out, it's possible! All you need is 150,000 times the pressure. OK that's hard. But science has found a shortcut. Here it is.
The researchers from Stanford stacked four thin sheets of graphite onto a platinum metal support structure and topped them with a bit of hydrogen. The team was actually trying to create a superior alternative to silicon but instead discovered they could turn graphite into diamond.
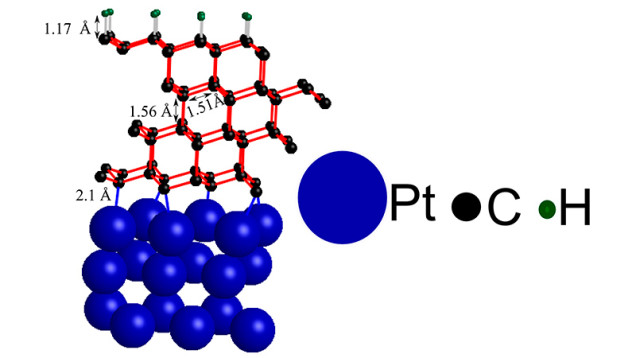
But while the restructured graphite behaves like diamonds, you can't bet on it to be on the finger of your lady friend anytime soon.
The diamonds produced using this method are more suited for industrial applications like coatings that make cutting blades sharper and more durable. [Stanford SLAC via Gizmag]
More Articles
Copyright © Fooyoh.com All rights reserved.